中文說明書 UNIONHONEST
本品僅供研究使用 酶聯免疫吸附測試 Enzyme linked lmmunosorbent Assay
昆蟲保幼激素(JH)ELISA檢測試劑盒
用 途: 用于昆蟲血清、血漿及相關液體樣本中保幼激素(JH)的測定。
檢測原理
本試劑盒采用的是生物素雙抗體夾心酶聯免疫吸附法(ELISA)測定樣品中昆蟲保幼激素(JH)的水平。向預先包被了本公司自己制備多克隆抗體-(可識別JH1/JH2/JH3)的酶標孔中加入保幼激素(JH),溫育;溫育后,加入生物素標記的抗保幼激素(JH) 抗體,再與鏈霉親和素-HRP結合,形成免疫復合物,再經過溫育和洗滌,去除未結合的酶,然后加入底物A、B,產生藍色,并在酸的作用下轉化成最終的黃色。顏色的深淺與樣品中昆蟲保幼激素(JH)的濃度呈正相關。
試劑盒組成
|
| 名 稱 | 96孔配置 12孔×8條 | 48孔配置 12孔×4條 | 備 注 |
| 1 | JH3標準品(240ng/ml) | 0.5ml | 0.5ml | 稀釋即用型 |
| 2 | 酶標包被板 | 1塊 | 1塊 | 已包被即用型 |
| 3 | 標準品稀釋液 | 3ml | 3ml | 即用型 |
| 4 | 鏈霉親和素-HRP | 6ml | 3ml | 即用型 |
| 5 | 30x倍濃縮洗滌液 | 20ml | 20ml | 蒸餾水稀釋 |
| 6 | 生物素標記的抗保幼激素(JH) 抗體 | 2ml | 2ml | 即用型 |
| 7 | 顯色劑A液 | 6ml | 3ml | 即用型 |
| 8 | 顯色劑B液 | 6ml | 3ml | 即用型 |
| 9 | 終止液 | 6ml | 3ml | 即用型 |
| 10 | 說明書 | 1份 | 1份 | 即用型 |
| 11 | 封板膜 | 2張 | 2張 | 不可反復使用 |
| 12 | 密封袋 | 1個 | 1個 | 即用型 |
JH3標準品來源:https://www.apexbio.cn/juvenile-hormone-iii.html
需要而未提供的試劑和器材
1. 酶標儀(450nm檢測波長濾光片,570nm或630nm校正波長濾光片) 。
2. 洗板機(可調注液量,保證每孔洗液加至0.35ml而不溢出)。
3. 超凈工作臺,生物安全柜,通風柜。
4. 高精度單道加液器(量程為0.5-10μl, 2-20μl,20-200μl,200-1000μl).
5. 高精度多道加液器(8道或12道,量程為50-300μl).
6. 37℃恒溫箱。
7. 低溫離心機。
8. 電冰箱(4℃,-20℃,-86℃).
9. 漩渦混合儀,低頻振蕩器等
注意事項
1. 從2-8℃取出的試劑盒,在開啟試劑盒之前要室溫平衡至少30分鐘。酶標包被板開封后如未用完,板條應裝入密封袋中保存。
2. 嚴格按照說明書的操作進行,試驗結果判定必須以酶標儀讀數為準。
3. 為避免交叉污染,要避免重復使用手中的吸頭和封板膜。
4. 不用的其它試劑應包裝好或蓋好。不同批號的試劑不要混用。保質前使用。
5. 底物B對光敏感,避免長時間暴露于光下。
6. 所有樣品,洗滌液和各種廢棄物都應按生物廢棄物處理。終止液為2M硫酸,使用時必須注意安全。
7. 各步驟加樣均應使用加樣器,并經常校準其準確性,以避免試驗誤差。一次加樣時間最好控制在5分鐘內,如標本數量多,推薦使用排槍加樣。
8. 每次試驗測定的同時做標準曲線,。如標本中待測物質含量過高(樣本OD值大于標準品孔最高濃度的OD值),請先用樣品稀釋液稀釋一定倍數(n倍)后再測定,計算時請最后乘以總稀釋倍數(×n)。
9. 配制試劑時,要用旋渦混合儀混勻。加入孔內的試劑,充分混勻對測試結果尤為重要,最好使用微量振蕩器(使用最低頻率),如無微量振蕩器,可在反應前手工輕輕晃動酶標板1分鐘,例如做圓周動作,使加入孔中的反應液混勻。
10. 實驗用酶標儀應嚴格按使用說明書規范操作,并在使用前充分預熱。
11. 手工洗板應注意:甩掉酶標板內的液體;在實驗臺上鋪墊幾層吸水紙,酶標板朝下用力拍幾次;將稀釋后的洗滌液至少0.35ml注入孔內,浸泡1-2分鐘。根據需要,重復此過程數次。自動洗板:如果有自動洗板機,應在熟練使用后再用到正式實驗過程中。
樣本要求
1.不能檢測含NaN3的樣品,因NaN3抑制辣根過氧化物酶的(HRP)活性。
2.標本采集后盡早進行提取,提取按相關文獻進行,提取后應盡快進行實驗。若不能馬上進行試驗,可將標本放于-20℃保存或根據存放,時間做相應溫度調整,但應避免反復凍融,(由于樣本種類過多,每個單位處理方式不一樣,本說明書不做詳細介紹)。
操作程序
1. 標準品的稀釋:(本試劑系統提供原倍標準品一支,用戶請按照說明自行在小試管中倍比稀釋)按下表格執行:
| 120ng/ml | (5號標準品) | 120μl的原倍標準品加入120μl的標準品稀釋液 |
| 60ng/ml | (4號標準品) | 120μl的5號標準品加入120μl的標準品稀釋液 |
| 30ng/ml | (3號標準品) | 120μl的4號標準品加入120μl的標準品稀釋液 |
| 15ng/ml | (2號標準品) | 120μl的3號標準品加入120μl的標準品稀釋液 |
| 7.5ng/ml | (1號標準品) | 120μl的2號標準品加入120μl的標準品稀釋液 |
2. 根據待測樣品數量加上標準品的數量決定所需的板條數。每個標準品和空白孔建議做復孔。每個樣品根據自己的數量來定,能使用復孔的盡量做復孔。
3. 加樣:1)空白孔:(空白對照孔不加樣品,生物素標記的抗保幼激素(JH)抗體,鏈霉親和素-HRP,其余各步操作相同 ;2)標準品孔:加入標準品50μl,鏈霉親和素-HRP50μl(標準品中已事先整合好生物素標記抗體,故在操作時不加,只加鏈霉素-HRP);3)樣本反應孔:加入樣本40μl,然后各加入抗保幼激素(JH)抗體10μl、鏈霉親和素-HRP(空白孔除外)50μl。蓋上封板膜,輕輕振蕩混勻,37℃溫育60分鐘。
4. 配液:將30倍濃縮洗滌液用蒸餾水30倍稀釋后備用。20倍濃縮洗滌液用蒸餾水20倍稀釋備用。
5. 洗滌:小心揭掉封板膜,棄去液體,甩干,每孔加滿洗滌液,靜置30秒后棄去,如此重復5次,拍干。
6. 顯色:每孔先加入顯色劑A 50μl,再加入顯色劑B 50μl,輕輕震蕩混勻,37℃避光顯色10分鐘。
7. 終止:每孔加終止液50μl,終止反應(此時藍色立轉黃色)。
8. 測定:以空白空調零,450nm波長依序測量各孔的吸光度(OD值)。 測定應在加終止液后10分鐘以內進行。
結果判斷
1. 每個標準品和標本的OD值應減去零孔的OD值。(若不減零孔值,標準曲線的零孔應相交于Y軸)
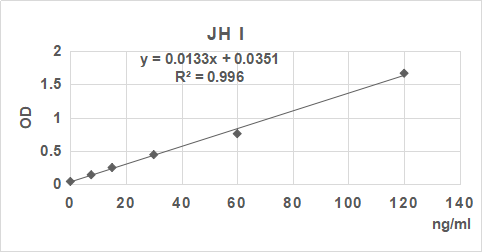
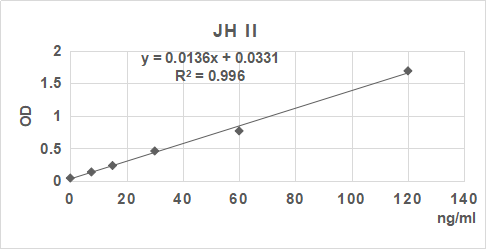
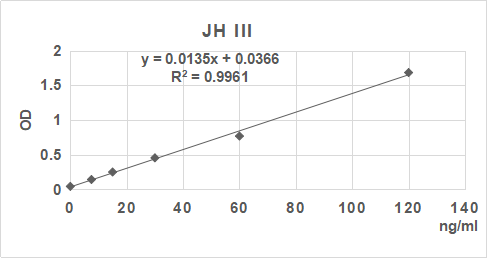
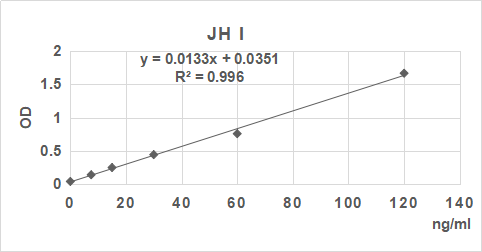
2. 根據標準品的濃度及對應的OD值計算出標準曲線的直線回歸方程,再根據樣品的OD值在回歸方程上計算出對應的樣品濃度。也可以使用各種熟悉應用軟件來計算。
3.手工繪制標準曲線。以標準品濃度作橫坐標,OD值作縱坐標,以平滑線連接各標準品的坐標點。通過標本的OD值可在標準曲線上查出其濃度。推薦使用專業制作曲線軟件進行分析計算結果。
4.若標本OD值高于標準曲線上限,應適當稀釋后重測,計算濃度時應乘以稀釋倍數。
| 0.045 | 0.144 | 0.252 | 0.456 | 0.77 | 1.685 |
|
| 0 | 7.5 | 15 | 30 | 60 | 120 | ng/ml |
| 0.047 | 0.14 | 0.239 | 0.462 | 0.77 | 1.691 |
|
| 0 | 7.5 | 15 | 30 | 60 | 120 | ng/ml |
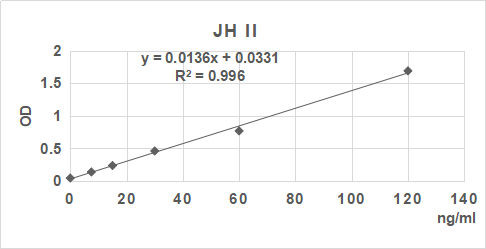
| 0.043 | 0.143 | 0.251 | 0.444 | 0.758 | 1.663 |
|
| 0 | 7.5 | 15 | 30 | 60 | 120 | ng/ml |
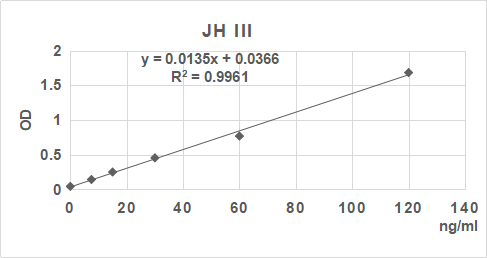
操作總結
準備試劑,樣品和標準品
![]()
加入準備好的樣品和標準品,生物素標記二抗和酶標試劑,37℃反應60分鐘。
![]()
洗板5次,加入顯色液A、B,37℃顯色10分鐘
![]()
加入TMB終止液
![]()
10分鐘內酶標儀測OD值
![]()
計算待測樣本因子含量
試劑盒性能
1. 準確性:標準品線性回歸與預期濃度相關系數R值,大于等于0.9200。
2. 靈敏度:最小可檢測濃度達,0.45ng/ml。
3. 特異性:不與其它可溶性結構類似物交叉反應。
4. 重復性:板內、板間變異系數均小于15%。
5 . 回收率:70-110%.
6. 貯藏:2-8℃,避光防潮保存。
7. 有效期:6個月
8. 檢測范圍:240ng/ml -1.875ng/ml
Insect juvenile hormone (JH) ELISA kit
Uses: For the determination of juvenile hormone (JH) in insect serum, plasma and related liquid samples.
Detection principle
This kit uses a biotin double antibody sandwich enzyme-linked immunosorbent assay (ELISA) to determine the level of insect juvenile hormone (JH) in the sample. Add juvenile hormone (JH) to the pre-coated polyclonal antibody (recognizing JH I/JH II/JH III) and incubate; after incubation, add biotin-labeled anti-juvenile hormone (JH) antibody, and then combined with streptavidin-HRP to form an immune complex, then incubate and wash to remove unbound enzymes, and then add substrates A and B to produce blue color, which is converted into the final yellow. The intensity of the color is positively correlated with the concentration of insect juvenile hormone (JH) in the sample.
Kit composition
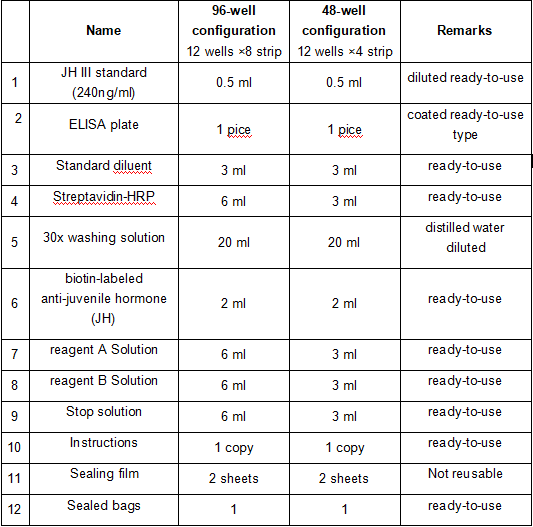
JH III standard:https://www.apexbio.cn/juvenile-hormone-iii.html
Materials required but not provided
1. Microplate reader (450 nm detection wavelength filter, 570 nm or 630 nm correction wavelength filter).
2. Plate washer (adjustable liquid injection volume to ensure that each well of washing liquid is added to 0.35 ml without overflow).
3. Ultra-clean workbench, biological safety cabinet, fume hood.
4. High-precision single-channel dosing device (range 0.5-10 μl, 2-20 μl, 20-200 μl, 200-1000 μl).
5. High-precision multi-channel dosing device (8 channels or 12 channels, 50-300 μl range).
6. 37℃ thermostat.
7. Low temperature centrifuge.
8. Refrigerator (4℃, -20℃, -86℃).
9. Vortex mixer, low frequency oscillator, etc.
Precautions
1. The kit taken out from 2-8℃ should be balanced at room temperature for at least 30 minutes before opening the kit. If the microliter plate is not used up after being unsealed by the plate, the lath should be stored in a sealed bag.
2. In strict accordance with the instructions, the test results must be judged by the readings of the Microplate reader.
3. In order to avoid cross-contamination, avoid reusing the pipette tips and sealing film.
4. Other reagents that are not used should be packed or covered. Do not mix different batches of reagents. Use before quality assurance.
5. Substrate B is sensitive to light to avoid prolonged exposure to light.
6. All samples, detergents and all kinds of wastes should be disposed of as biological wastes. The terminating solution is 2M sulfuric acid, and safety must be paid attention to when using it.
7. Samplers should be used in each step, and their accuracy should be calibrated frequently to avoid test errors. The time of adding a sample should be controlled within 5 minutes. If there are a large number of specimens, it is recommended to use pipette to add samples.
8. Make the standard curve at the same time of each test. If the content of the substance to be tested in the sample is too high (the OD value of the sample is greater than the OD value of the highest concentration of the standard hole), please first dilute the sample diluent by a certain multiple (n times) before determining it, and then multiply the total dilution multiple (× n) at last.
9. When preparing reagents, use a vortex mixer to mix well. The reagent added into the well is particularly important for the test results. It is best to use a trace oscillator (use the lowest frequency). If there is no trace oscillator, you can gently shake the elisa plate by hand for 1 minute before the reaction, such as circular movement, to mix the reaction solution in the well.
10. The Microplate reader for the experiment should operate strictly according to the operating instructions and be fully preheated before use.
11. Manual washing plate should pay attention to: get rid of the liquid in the elisa plate; lay several layers of absorbent paper on the test bench, and slap the plate down several times; at least pour the diluted detergent into the well and soak for 1-2 minutes. Repeat this process several times as needed. Automatic washing machine: if there is an automatic plate washing machine, it should be used in the formal experimental process after being proficient in use.
Sample requirement
1. The sample containing NaN3 could not be detected because NaN3 inhibited the (HRP) activity of horseradish peroxidase.
2. The samples should be extracted as soon as possible after collection, and the extraction should be carried out according to the relevant literature. If the test cannot be carried out immediately, the specimen can be stored at -20℃ or stored according to the storage time, and the corresponding temperature can be adjusted, but repeated freezing and thawing should be avoided.
Assy procedure
1. Dilution of the standard: (this reagent system provides one standard; users should follow the instructions to dilute the standard in a small test tube by themselves.) follow the table below:
| 120 ng/ml | (standard 5) | The original standard of 120 μ l is added to the diluent of 120 μ l of the standard. |
| 60 ng/ml | (standard 4) | 120 μ l of standard 5 is added to 120 μ l of standard dilution. |
| 30 ng/ml | (standard 3) | 120 μ l of standard 4 is added to 120 μ l of standard dilution. |
| 15 ng/ml | (standard 2) | 120 μ l of standard 3 is added to 120 μ l of standard dilution. |
| 7.5 ng/ml | (standard 1) | 120 μ l of standard 2 is added to 120 μ l of standard dilution. |
2. Determine the number of slats required according to the number of samples to be tested plus the number of standards. Double wells are recommended for each standard and blank well. Each sample is determined according to its own quantity, and multiple wells can be used as much as possible.
3. Add sample: 1) blank well: (blank well without sample, biotin-labeled anti-juvenile hormone (JH) antibody, streptavidin-HRP, other steps are the same; 2) standard well: add standard 50 μl, streptavidin-HRP 50 μl (biotin-labeled antibody has been integrated in advance in the standard, so do not add in the operation, only add streptomycin-HRP). 3) sample reaction well: add sample 40 μl, then add anti-juvenile hormone (JH) antibody 10 μl, streptavidin-HRP (except blank well) 50 μl. Cover the sealing film, gently shake and mix well, incubate at 37℃ for 60 minutes.
4. Mix liquid: wash solution (30x) will be diluted 30 times with distilled water. The wash solution (20x) diluted 20 times with distilled water.
5. Washing: carefully remove the sealing film, discard the liquid, shake dry, fill each hole with detergent, rest for 30 seconds and then discard, repeat 5 times, pat dry.
6. Color development: add chromogenic agent A 50 μl to each well, and then add chromogenic agent B 50 μl, gently shake and mix well, and avoid light at 37℃ for 10 minutes.
7. Termination: add 50 μl of terminating liquid to each hole to terminate the reaction (at this time, blue turns to yellow).
8. Measurement: the absorbance (OD value) of each well was measured sequentially with zero blank air conditioner and 450 nm wavelength. The determination should be carried out within 10 minutes after adding the terminator.
Result judgment
1. The OD value of each standard and specimen should be subtracted from the OD value of the zero well. (if the zero well value is not reduced, the zero well of the standard curve should intersect on the Y axis).
2. The linear regression equation of the standard curve is calculated according to the concentration of the standard sample and the corresponding OD value, and then the corresponding sample concentration is calculated on the regression equation according to the OD value of the sample. You can also use a variety of familiar applications to calculate.
3. Standard curve: The concentration of the standard is used as the abscissa, the OD value as the longitudinal coordinate, and the smooth line is used to connect the coordinate points of each standard. The OD value of the sample can be used to find out its concentration on the standard curve. It is recommended to use professional curve software to analyze and calculate the results.
4. If the OD value of the sample is higher than the upper limit of the standard curve, it should be diluted properly and retested, and the concentration should be multiplied by dilution multiple.
Operation summary
1. Prepare reagents, samples and standards.
2. The prepared samples and standards were added, and the biotin-labeled secondary antibodies and enzyme-labeled reagents were reacted at 37℃ for 60 minutes.
3. Wash plate 5 times and add chromogenic solution A and B to develop color at 37℃ for 10 minutes.
4. Add TMB Terminator.
5. OD value measured by Microplate reader within 10 minutes.
6. Calculate the factor content of the sample to be tested.
Kit performance
1. Accuracy: the correlation coefficient R between standard substance linear regression and expected concentration is greater than or equal to 0.9200.
2. Sensitivity: minimum detectable concentration, 0.45 ng/ml.
3. Specificity: does not cross-react with other soluble structural analogues.
4. Repeatability: the coefficient of variation within and between plates is less than 15%.
5. Recovery rate: 70-110%.
6. Storage: 2-8℃, keep away from light and moisture.
7. Validity period: 6 months.
8. Detection range: 240 ng/ml-1.875 ng/ml
9. Standard curve: